




On February 20th 2026, the U.S. Supreme Court struck down tariffs imposed under the International Emergency Economic Powers Act (IEEPA), holding that Congress did not delegate authority to the President to levy tariffs through that statute. The Court affirmed lower‑court rulings that invalidated...

On February 5, 2026, the administration launched TrumpRx, an online platform designed to help cash‑paying patients access prescription drugs at discounted prices. TrumpRx does not dispense medications directly but connects consumers to...
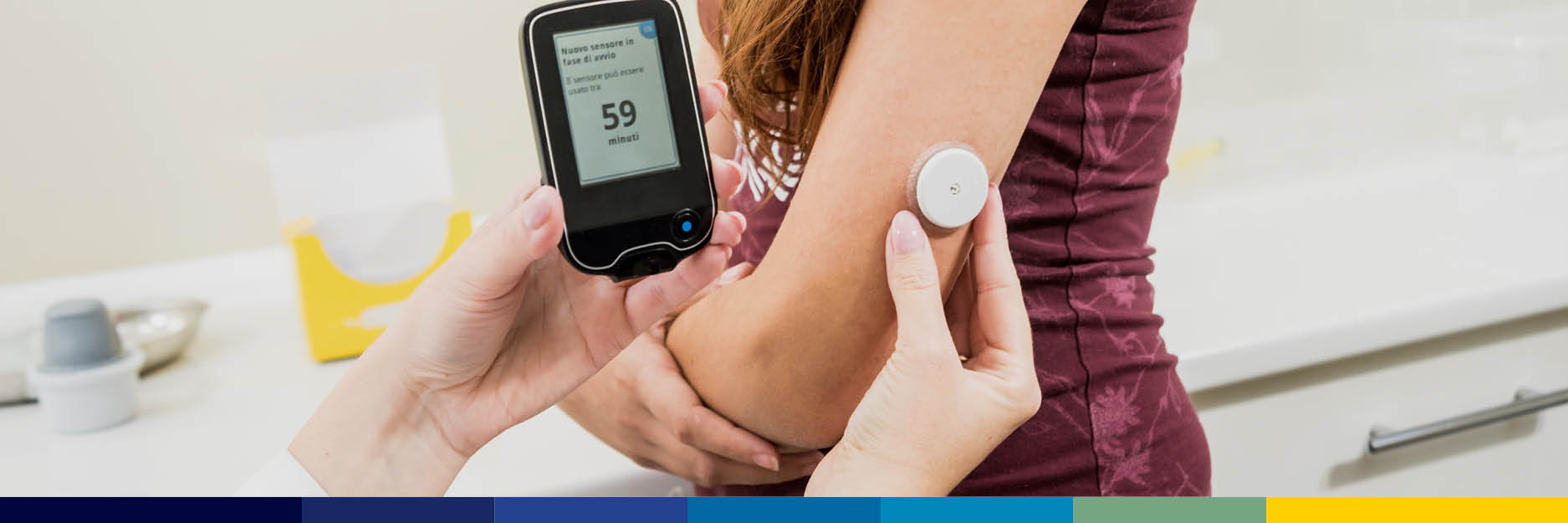
Diabetes technology is evolving faster than ever, and insulin pumps – once used almost exclusively in type 1 diabetes – are now playing a major role across type 2 diabetes (T2D) as well. As automated insulin delivery (AID) systems become more...

Medication therapy works best when it’s paired with ongoing lifestyle support in obesity care – especially when we’re talking about GLP‑1s. The Omada Insights Lab evaluated outcomes in the Enhanced GLP-1 Care Track at one year, and found...

Eli Lilly has announced a major policy change for participants in the 340B Drug Pricing Program, effective February 1, 2026. Moving forward, covered entities will be required to submit claims data for all Lilly drugs dispensed through in‑house pharmacies...





