



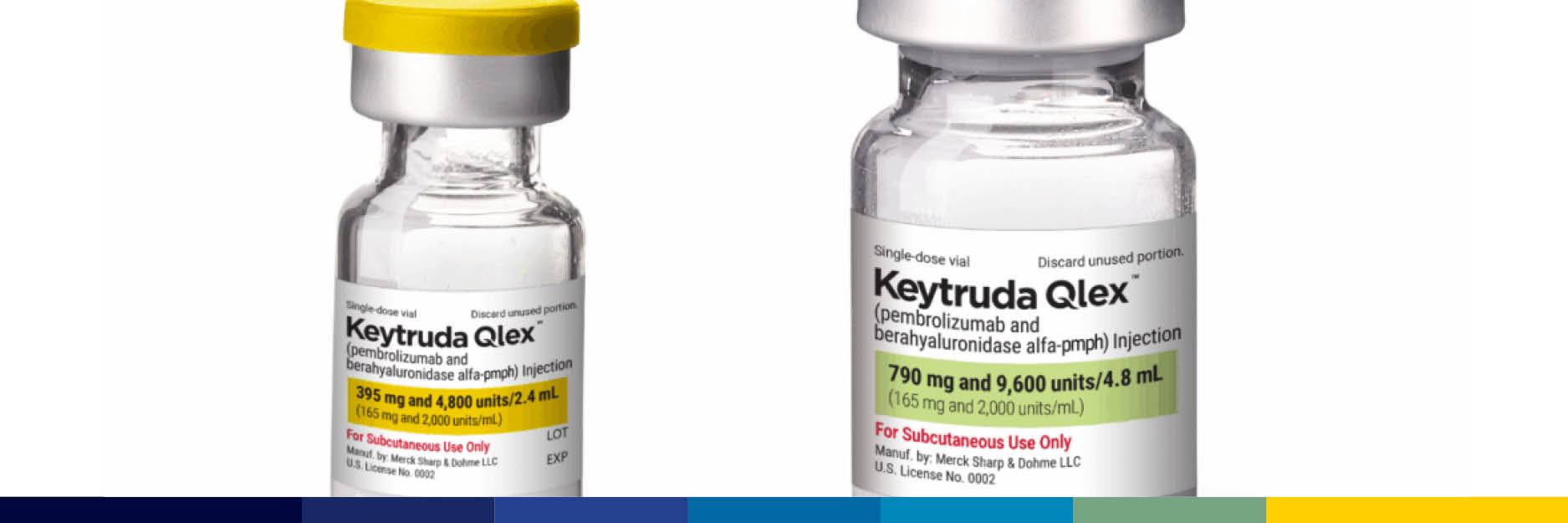
As several companies advance in developing biosimilars for Keytruda, Merck & Co. has introduced Keytruda Qlex – a subcutaneous formulation of their leading cancer therapy. Industry analysts suggest this innovative launch is strategically positioned to...

EMD Serono is the third manufacturer to partner with the Trump administration on “Most Favored Nation” (MFN) pricing – following earlier agreements with Pfizer and AstraZeneca (AZ). Similar to the Pfizer and AZ agreements, EMD Serono has agreed to offer...

Through ProAct's Advantage and Core formulary options, we use our unmatched scale and proven track record to advocate on our clients behalf, leveraging negotiations to provide competitive pricing. When clinical considerations align with these opportunities, patients and payers benefit...

On September 30, 2025, the Trump administration announced that Pfizer will provide its drugs at most favored nation (MFN) pricing, this is the lowest price paid by developed nations, to Medicaid programs. Pfizer will also provide certain medications direct to cash-paying consumers at a discount through a new website...

According to the latest National Alliance of Healthcare Purchaser Coalitions survey, 31% of U.S. employers have chosen a transparent pharmacy benefit manager (PBM) – that's up from 12% in 2024. This increase reflects a major industry shift. In just one year, employer contracts with the “Big Three” PBMs...





