
Welcome back. In this edition of Ron’s Clinical Corner, I would like to give a brief overview and update on new advances in the treatment of cystic fibrosis.
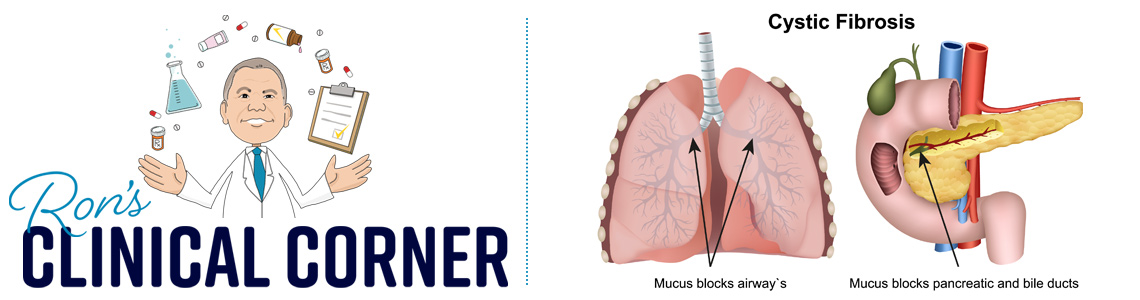
Cystic fibrosis (CF) is a genetic disorder characterized by an abnormality in chloride transport resulting in impaired clearance of secretions and mucus from the lungs, small intestine, liver, and pancreas. The disease most commonly results in symptoms in the lung (e.g. infections, decreased function) and pancreatic insufficiency. Historically, patients would be treated with supplemental pancreatic enzymes (to improve fat, protein, and carbohydrate metabolism), along with inhaled or systemic antibiotics and mucolytics to assist with improving lung function.
In 2012, the first cystic fibrosis transmembrane conductance regulator (CFTR) modulator, Kalydeco® was approved. Following approval of Kalydeco®, two additional medications, Orkambi® and Symdeko® came to market. These medications have been approved only for a subset of patients where there is homozygous F508del mutation or where clinical evidence supported use for another given CFTR mutation. Taking all of these things into account, the medications targeted genetic mutations found in 50% or less of cystic fibrosis patients.
Recently, an additional CFTR modulator, Trikafta® was approved. Not only does this medication appear to be more effective than the previously approved CFTR therapies, it is also indicated for a more expansive cystic fibrosis population. The labeled indications for Trikafta® support use in patients who have at least one F508del mutation in the CFTR gene.
Because all of these medications target the genetic defect that causes CF, they actually modify the disease process and lead to improvement. Unfortunately, many patients will have residual damage in their lungs and other organs due to what the disease has done before treatment. Furthermore, studies show that in order for patients to continue to see benefit from the CFTR modulators, they must be continued indefinitely. With an estimated annual cost of $300,000 for treatment with a CFTR modulator, this could drive significant cost for health plans who have a member(s) receiving treatment for cystic fibrosis.
Thanks for stopping in and see you next month.





